Engineering Microtissues for Development and Disease
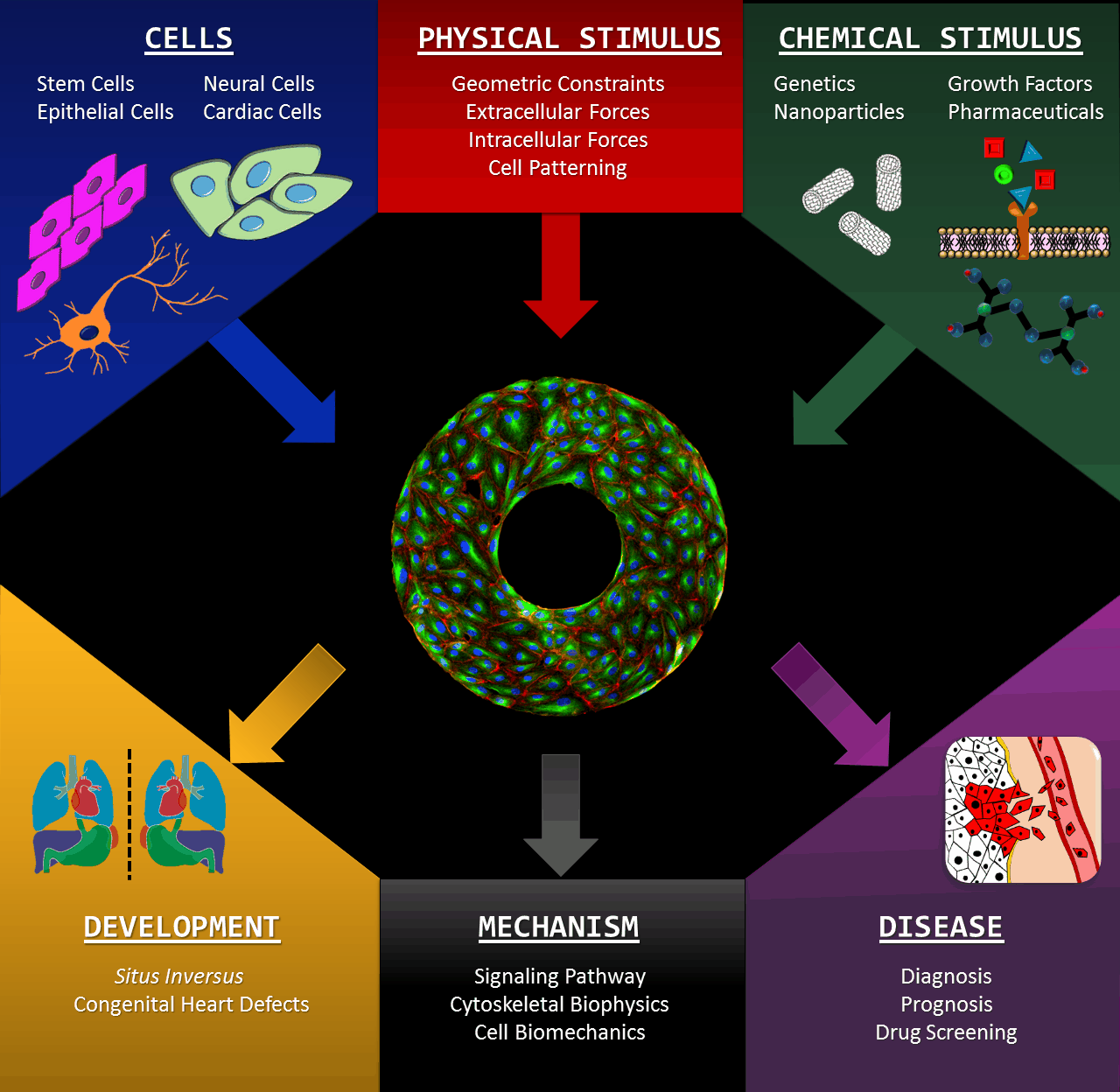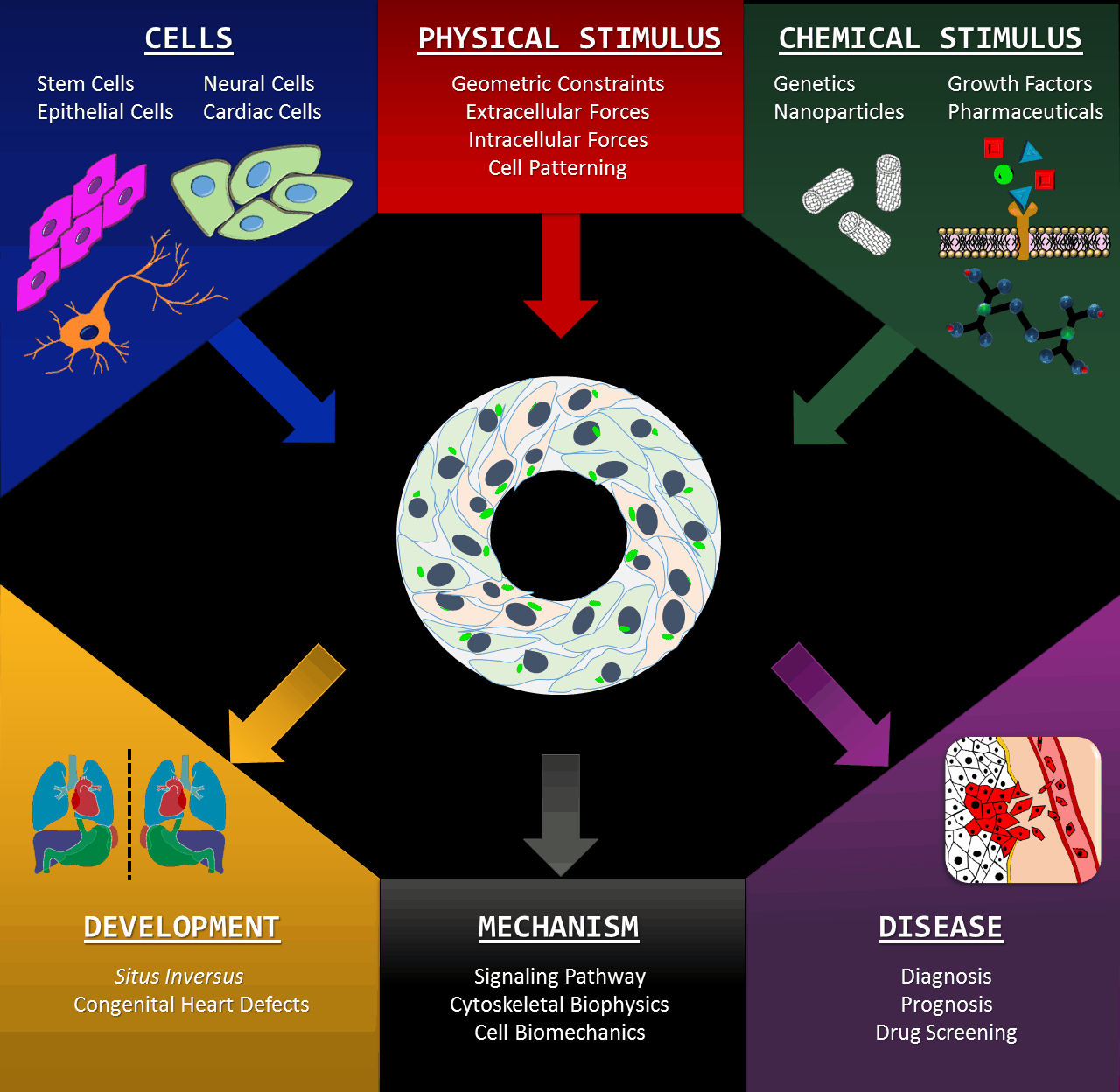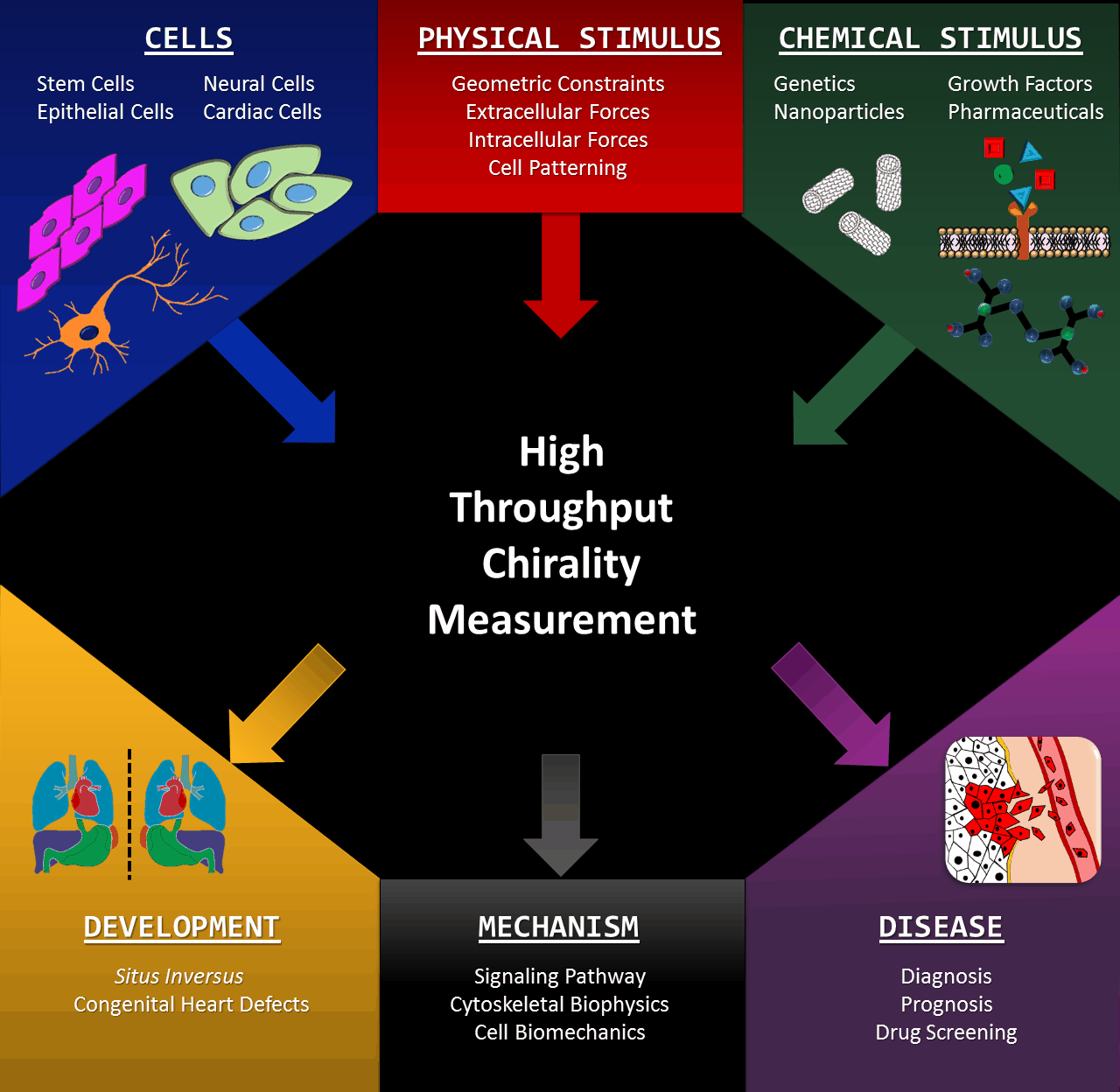
Microscale biomimetic tissue models can provide deep insights into mechanisms of tissue development and diseases. Our innovative in vitro models allow scientisists to look into the left-right (LR) asymmetry at a cellular level, which is also known as handedness or chirality (click here for a TED-Ed video lectured by Dr. Leo Wan). Cell chirality is a fundamental and ubiquitous characteristic inherent to a broad spectrum of living organisms: manifestations ranging from the twining of climbing plant vines to the helices of snail and conch shell. The formation and positioning of the heart and other organs are quintessential examples of bilateral asymmetry in the human body. We are developing novel in vitro modalities for studying cellular LR asymmetry and chiral morphogenesis, based upon the orientation, morphology and directional migration of cells cultured on micro-patterns. This intuitive system provides significant advantages including high-throughput and excellent imaging-compatibility. We are utilizing the assets of our micro-scale systems to identity the molecular mechanisms associated with the establishment of left-right asymmetry. Our in vitro studies are expanding into three dimensional systems catalyzing a deeper understanding of the complexity underlying in vivo tissue morphogenesis in early development as well as the progression of disease.
For more information, please refer to the "Introduction to Cell Chirality" video that previous lab student Tony Certo made for the lab's outreach program. Thanks to Tony for his work; we hope that it will help your understanding of our chirality project.
Functional Tissue Engineering and Cartilage Repair
Osteoarthritis (OA) is a degenerative joint condition that affects approximately 27 million Americans. Current diagnostic modalities are inadequate, as they cannot identify degeneration until severe damage has been done. This limits the use of early treatments to slow disease progression and leaves only aggressive treatments, such as joint arthroplasty, as viable options. Additionally, cartilage has a very limited ability to self-repair, necessitating novel stem cell-based techniques for the regeneration of functional neo-cartilage. Our lab is interested both in early diagnosis of OA and engineering cartilage for regenerative purposes.
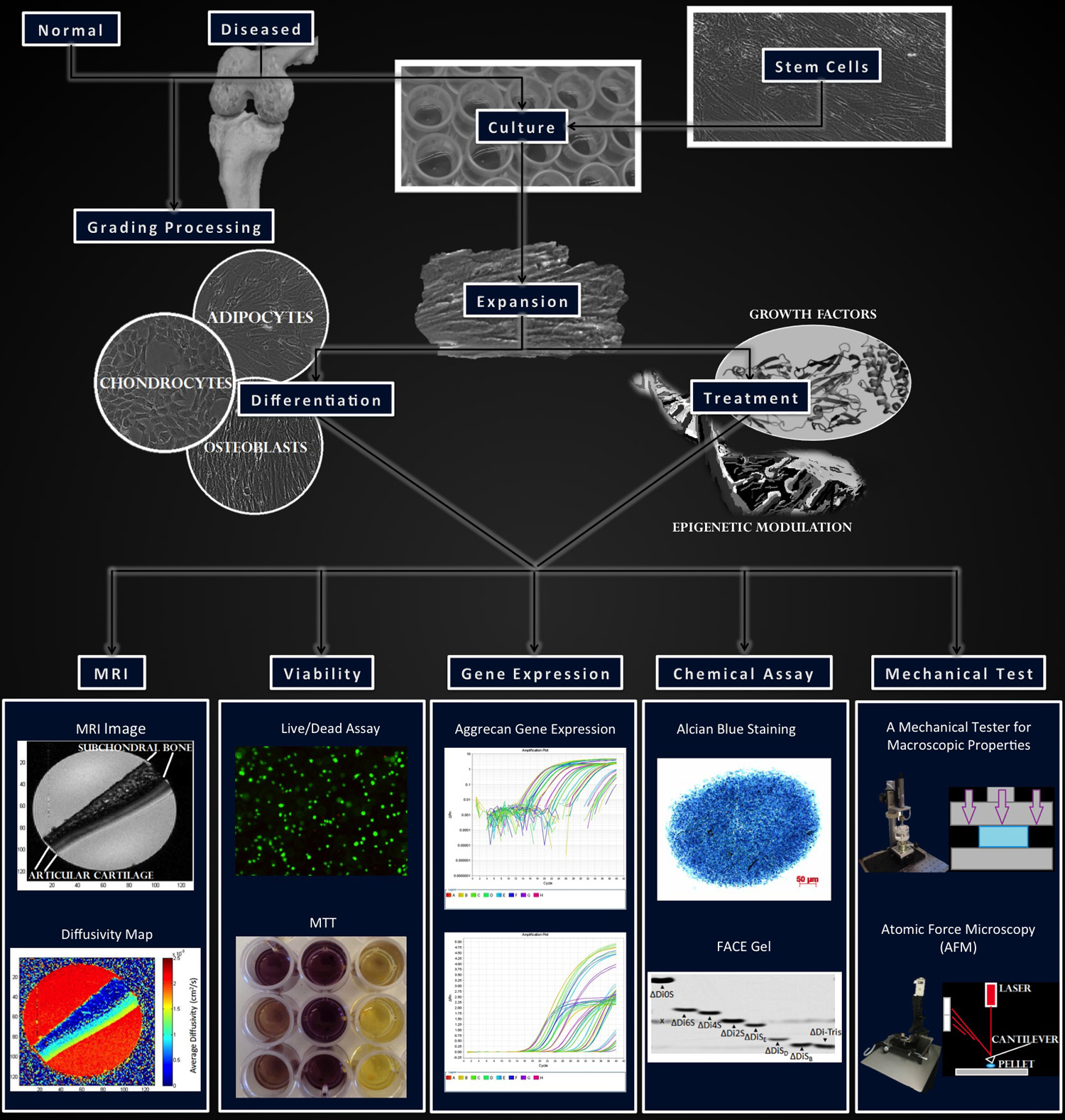
Using a broad array of techniques including imaging, mechanical testing, and biochemical assays, our research focuses on characterizing differences in healthy, diseased, and engineered articular cartilage. Research on normal and diseased cartilage and chondrocytes provides deep insight on genetic and epigenetic mechanisms associated with OA pathology, and serve as a guideline for stem cell based cartilage repair. Changes in anabolic (collagen and glycosaminoglycan production) and catabolic (matrix degradation or remodeling) molecular interplay in normal and OA cartilage can lead to alterations in tissue structure and function. Cellular responses to differential molecular treatments using our high throughput methods will provide insight on the disease-modifying capabilities of potential drugs on native chondrocytes and implanted stem cells. Our findings will aid in the development of stem cell based methods for modeling cartilage in development and disease.